January 4th, 2018
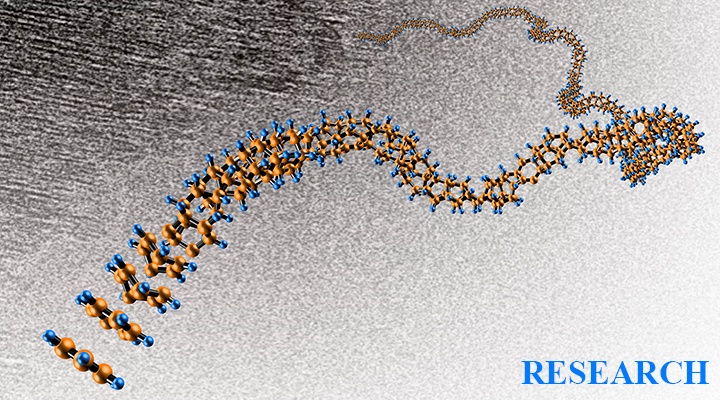
EFree theorists led by Postdoctoral Associate Hanyu Liu predicted that by tailoring the pressure and composition of a mixture of hydrogen and rare-earth elements, exotic metals would be created, some of which would remain superconducting to nearly room temperature. Their results were published in H. Liu et al., Proc. Natl. Acad. Sci. USA...
October 20th, 2017
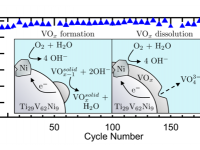
Over the past several decades, the current state-of-the-art alloys for metal hydride (MH) batteries have been LaNi5 based AB5 materials with a reversible capacity of 300 mAh/g. Since the advent of MH batteries, researchers have been searching for early transition metal hydrides to replace AB5 alloys. Replacing the heavy AB5 alloys with...
October 20th, 2017
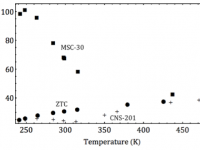
A fast, reversible, and energy efficient sorbent for the separation of carbon dioxide from mixed gas streams has been sought for energy applications in purifying flue gas and upgrading biogas. Biogas is a methane-rich gas evolved from decomposing organic matter at farms, landfills and wastewater treatment plants. While thousands of...
September 21st, 2017
Extreme conditions research cannot be accomplished without state-of-the-art instruments. Thus, tools must be improved in order to access new domains and to expand the range of properties measured. In high pressure neutron diffraction, for example, one limitation that sample sizes need to be much larger than is the case for x-ray...
August 11th, 2017
The 2017 EFRC PI meeting was held at the Marriott Wardman Park Hotel in Washington DC on July 24-25. After plenary lectures on the possibilities for exascale computing and the future of quantum information science, the meeting featured highlight presentations from each of the EFRC, CMS and Hub directors, along with technical talks and...