 Over the past several decades, the current state-of-the-art alloys for metal hydride (MH) batteries have been LaNi5 based AB5 materials with a reversible capacity of 300 mAh/g. Since the advent of MH batteries, researchers have been searching for early transition metal hydrides to replace AB5 alloys. Replacing the heavy AB5 alloys with lightweight transition metal alloys could double or even triple the capacity of the anode. In an electrochemical setting, however, these early transition metal based electrodes suffer from poor cycle life. Only recently did a Japanese group achieve a discharge capacity greater than 400 mAh/g for 30 cycles with a Ti-V-Ni-Cr alloy.
Over the past several decades, the current state-of-the-art alloys for metal hydride (MH) batteries have been LaNi5 based AB5 materials with a reversible capacity of 300 mAh/g. Since the advent of MH batteries, researchers have been searching for early transition metal hydrides to replace AB5 alloys. Replacing the heavy AB5 alloys with lightweight transition metal alloys could double or even triple the capacity of the anode. In an electrochemical setting, however, these early transition metal based electrodes suffer from poor cycle life. Only recently did a Japanese group achieve a discharge capacity greater than 400 mAh/g for 30 cycles with a Ti-V-Ni-Cr alloy.
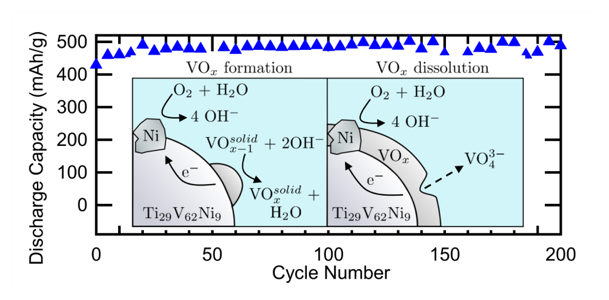
The Fultz group at Caltech used the Pourbaix diagram for V as a guide in developing a strategy to slow the corrosion of V-based BCC electrodes. A Pourbaix diagram plots the stable phases of an aqueous electrochemical system; at the pH and electrochemical potential associated with MH batteries the stable phase of V is the aqueous (VO4)3- anion (oxidation state of +5). The researchers found that removing oxygen from the system is critical to improve the cycle life of V-based BCC MH electrodes. The cycle life is further improved by either vanadate ion additions to the electrolyte or Cr substitutions in the alloy. Figure 1 plots the cycling data for a sealed button cell containing a Ti29V62Ni9 electrode and KOH electrolyte with 50 mM KVO3 added. A discharge capacity of 500 mAh/g is achieved for over 200 cycles, and up to 600 mAh/g with a shortened cycle life. The schematic in Fig. 1 illustrates the redox reactions that occur on the surface of the MH particles which result in a gradual capacity fade. Eliminating oxygen from the system and adding vanadate ions to the electrolyte will drastically slow the reactions to the point that long term cycling is achieved.
This is an important result because it provides a new family of materials and new strageties for advancing MH batteries. An engineering study in collaboration with JPL showed that with an air electrode, a MH-air battery utilizing these alloy electrodes can achieve an energy density comparable to state-of-the-art Li-ion batteries.
Caption: Cycling data for a button cell containing Ti29V62Ni9 electrodes and KOH electrolyte with 50 mM KVO3 added. The inset schematic depicts the redox reactions that occur during the process of V corrosion and dissolution into the electrolyte.
