 Goal: To characterize the structural features that underlie ion transport in battery materials.
Goal: To characterize the structural features that underlie ion transport in battery materials.
Personnel: Brent Fultz (Lead), Chen Li, Sally Tracy
Project Documents: EFree Papers, Relevant Publications, Progress Reports
Overview: Transport of electrons or ions is a central issue in many energy storage materials. In particular, battery cathode materials require both mobile ions and electronic conductivity via small polaron hopping. Measurements of the temperature and pressure dependence of the charge hopping frequency allow for the determination of an activation volume, and this quantity can provide a window into the atomic rearrangements that occur during the transient state of a hopping event.
Transport processes can usually be understood as activated processes. The activation energy is the energetic barrier for the moving species to hop between adjacent sites, and the activation volume quantifies the effect of pressure on this activation barrier, as it gives the local change in volume as the particle moves through its transition state. The important role of activation energy in setting the temperature dependence of the transport process is well known, but the activation volume is less well understood. Pressure is a unique tool for measuring transient distortions that occur in a crystal during the motion of an ion or electron, and in EFree, we are using pressure to measure the activation volume for the transport of charge or ions in battery cathode materials.
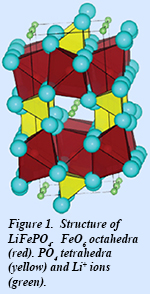
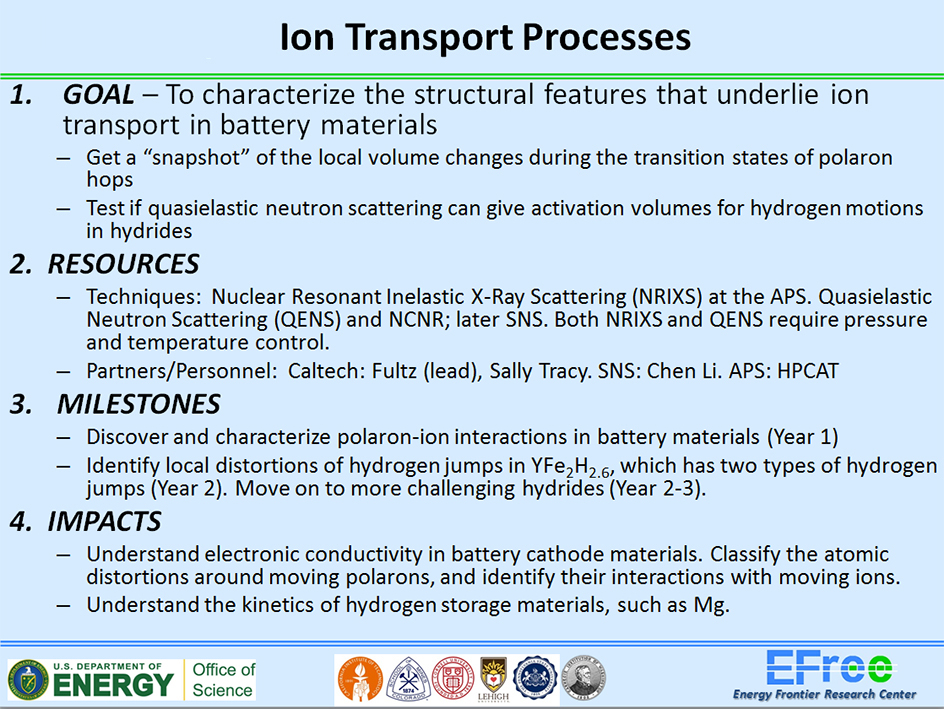
During the first phase of EFree, we studied the activation volume (Va) for polaron hopping in LixFePO4 (Fig. 1). In this material, thermal vibrations can distort the local environment between adjacent Fe2+ and Fe3+ ions to help the electron hop from the Fe2+ and Fe3+ site. The specific role of applied pressure in this case is to change these jump rates and allow us to deduce the size of the local distortion required for this charge transfer to occur. Using synchrotron nuclear forward scattering (NFS) spectroscopic techniques that we have developed for studying this problem (Fig. 2), we found a strong suppression of the polaron hopping rate with pressure, which is quite atypical of other polaron conductors reported thus far. Based on this data, we found Va = +6 Å3, which is characteristic of Li-ion diffusivity, and suggests a correlated dynamics of the polarons and Li-ions. The phenomenon extends traditional polaron physics to include a coupling between a polaron quasiparticle and a classical particle (the Li+ ion), which alters the dynamics of both. This is a new physical process, with only hints in the previous literature, but it is in fact essential to understanding the electrochemical performance of LixFePO4.
We are now completing a study of how Va depends on the Li concentration. We are also preparing a study on a related material, NaxFePO4. The size discrepancy between Na and Li ions will likely affect the activation volume for both ion and polaron hopping.
The one-dimensional character of the ion pathways in LiFePO4 results in an ion mobility that can be impeded by defects that block the channels of ion transport. Blocked channels for Li-ions then suppress electronic conductivity if polaron-ion interactions are strong. Li2FeSiO4 has two-dimensional channels for ion conduction, so the polaron-ion interactions may well be different.
