 The sodium counterpart to LiFePO4 has recently attracted attention as a potentially promising cathode material. Sodium is one of the major elements in the Earth’s crust and is both environmentally benign and affordable. As a result, the development of sodium analogues to lithium cathode materials is appealing, especially for large energy-storage systems.
The sodium counterpart to LiFePO4 has recently attracted attention as a potentially promising cathode material. Sodium is one of the major elements in the Earth’s crust and is both environmentally benign and affordable. As a result, the development of sodium analogues to lithium cathode materials is appealing, especially for large energy-storage systems.
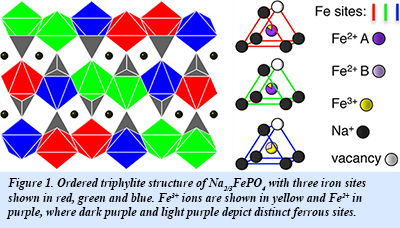
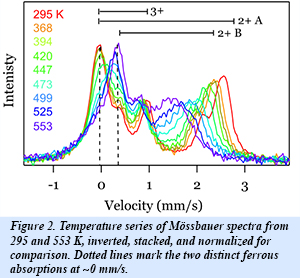 The interplay between sodium ordering and electron mobility in NaxFePO4 (Fig. 1) was investigated using a combination of synchrotron x-ray diffraction and Mössbauer spectrometry. These results provide the temperature of sodium redistribution on the lattice and yield new information about the phase stability of the system. An analysis of the temperature evolution the iron site occupancies from the Mössbauer measurements (Fig. 2) was used to identify the onset of fast electron hopping. These results combined with synchrotron x-ray diffraction, show a relationship between the onset of electron dynamics and the loss of local order on the sodium sublattice. These types of coupled processes may be common to many materials used for battery electrodes, and new details concerning the influence of polaron-ion interactions on the charge dynamics are relevant to optimizing their electrochemical performance [S. J. Tracy et al., Chem. Mater. 28, 3051-3059 (2016)].
The interplay between sodium ordering and electron mobility in NaxFePO4 (Fig. 1) was investigated using a combination of synchrotron x-ray diffraction and Mössbauer spectrometry. These results provide the temperature of sodium redistribution on the lattice and yield new information about the phase stability of the system. An analysis of the temperature evolution the iron site occupancies from the Mössbauer measurements (Fig. 2) was used to identify the onset of fast electron hopping. These results combined with synchrotron x-ray diffraction, show a relationship between the onset of electron dynamics and the loss of local order on the sodium sublattice. These types of coupled processes may be common to many materials used for battery electrodes, and new details concerning the influence of polaron-ion interactions on the charge dynamics are relevant to optimizing their electrochemical performance [S. J. Tracy et al., Chem. Mater. 28, 3051-3059 (2016)].
