 The band structure of a material is determined by the electronic configuration of its components and their arrangement symmetry. Pressure is known to be able to alter the electronic configuration and atomic arrangements, and therefore change the band structure. A team of researchers led by Shibing Wang and Wendy L. Mao (Stanford University) discovered that in CsAuI3, a mixed valence compound with Au1+ and Au3+, the bandgap first closes with pressure and then opens up again due to a first-order structural transition, a rare occasion in transition metal compounds.
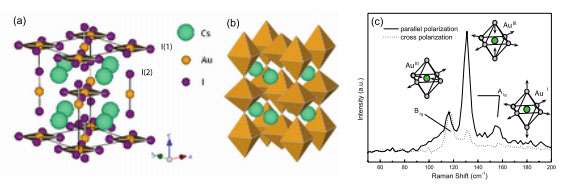
The band structure of a material is determined by the electronic configuration of its components and their arrangement symmetry. Pressure is known to be able to alter the electronic configuration and atomic arrangements, and therefore change the band structure. A team of researchers led by Shibing Wang and Wendy L. Mao (Stanford University) discovered that in CsAuI3, a mixed valence compound with Au1+ and Au3+, the bandgap first closes with pressure and then opens up again due to a first-order structural transition, a rare occasion in transition metal compounds.CsAuI3 is a representative of a family of unique 5d transition metal compounds that have a ground state with dn-1 and dn+1 electronic configurations. Pressure induces a mixed-valence to single-valence transition through a structural transition at approximately 5.5 GPa. In their earlier study [S. Wang et al. Phys. Rev. B, 87, 054104 (2013)], the group discovered that under hydrostatic compression the system undergoes a tetragonal to orthorhombic transition. In their current work, they present how the bonding and band structure of CsAuI3 change at high pressure. Experimental Raman spectroscopy and theoretical calculation provide detailed information on the changes of the bonding along the valence transition. For the first time IR spectroscopy, which was conducted at the U2A beamline at the National Synchrotron Light Source, shows the closing of the charge-density-wave gap at high pressure, and provides direct evidence of the insulating nature of the high-pressure orthorhombic single-valence phase.
The study provides crucial insights into the interplay between atomic arrangements and electronic configuration and reveals how they work together to alter the band structure, offering a new avenue for bandgap engineering [S. Wang et al., Phys. Rev. B. 89, 245109 (2014)].
