 Methane is a proven fuel, and its importance will grow with new sources of supply. The storage of methane gas for mobile vehicles could benefit from increased volumetric energy density at a given pressure. One approach is physisorption, a relatively weak, non-chemical binding of molecules to the surface of an adsorbent material. The quantity of methane adsorbed increases with the surface area of the adsorbent material (number of adsorption sites) and the strength of binding at each site (proportional to the isosteric enthalpy of adsorption). Low-cost physisorption materials have already been added to storage tanks for increasing the volumetric energy density of methane, but their effects have been modest.
Methane is a proven fuel, and its importance will grow with new sources of supply. The storage of methane gas for mobile vehicles could benefit from increased volumetric energy density at a given pressure. One approach is physisorption, a relatively weak, non-chemical binding of molecules to the surface of an adsorbent material. The quantity of methane adsorbed increases with the surface area of the adsorbent material (number of adsorption sites) and the strength of binding at each site (proportional to the isosteric enthalpy of adsorption). Low-cost physisorption materials have already been added to storage tanks for increasing the volumetric energy density of methane, but their effects have been modest.Previous adsorbents have consistently shown isosteric enthalpies of adsorption that decrease with methane coverage on the surface. Intuitively, for a surface with heterogeneous binding sites, the most favorable sites are occupied first, and the isosteric enthalpy of adsorption decreases with more coverage.
With EFree support, Caltech researchers Nicholas Stadie, Maxwell Murialdo, Channing Ahn, and Brent Fultz have shown that through careful control of the pore size distribution of the adsorbent material, the isosteric enthalpy of adsorption can be made to increase as a function of loading. This is an important step toward realizing practical adsorptive methane storage near ambient conditions - a key topic of future energy storage for mobile applications.
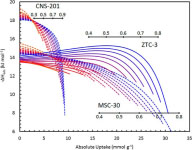
This was realized experimentally in a zeolite-templated carbon with a narrow pore-size distribution centered at 1.2 nm. At pressures of tens of atmospheres, the confinement of methane molecules within the pores promoted attractive methane-methane intermolecular interactions, causing an increased isosteric enthalpy of adsorption as more sites were occupied. Gas densification at high pressures is enhanced.
This discovery suggests a general approach for using the pore-size distributions of microporous materials to tune the thermodynamic properties of adsorption at elevated pressures. Researchers used non-ideal gas behavior with appropriate surface structures, with a result that nanostructured materials could be designed with tuned physisorption thermodynamics, optimized for storage of methane and other non-ideal gases. Already, a zeolite-templated carbon showed one of the highest quantities of methane adsorption reported to date. Furthermore, the shape of its isosteric heat with coverage leads to a higher delivery of methane from a storage tank that operates between two finite pressures [N. P. Stadie, et al., J. Am. Chem. Soc. 135, 990-993 (2013); N. Stadie, et al., provisional pattent application # 61/716,041 (2012)].
Figure Caption: Isosteric enthalpies of methane adsorption by three materials at several temperatures. The zeolite-templated carbon ZTC-3 shows an anomalous increase with uptake.
