 Compact hydrogen storage that is effective and practical remains an important obstacle to realizing a hydrogen energy economy. Physisorption of hydrogen on the surface of an adsorbent material is a promising solution. High pressure can be used to reach reasonable hydrogen densities, but few thermodynamic studies of hydrogen physisorption have been performed above 10 MPa due to a lack of instruments capable of accurately measuring hydrogen sorption at these pressures. With EFree support, Caltech researchers Nicholas Stadie, Brent Fultz, and their collaborators designed, constructed, and commissioned such an apparatus for thermodynamic gas adsorption measurements up to 70 MPa, a relevant target pressure for mobile storage applications.
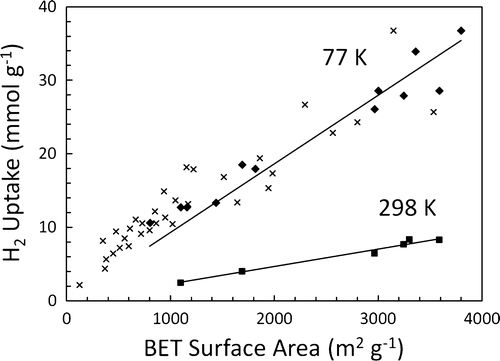
Compact hydrogen storage that is effective and practical remains an important obstacle to realizing a hydrogen energy economy. Physisorption of hydrogen on the surface of an adsorbent material is a promising solution. High pressure can be used to reach reasonable hydrogen densities, but few thermodynamic studies of hydrogen physisorption have been performed above 10 MPa due to a lack of instruments capable of accurately measuring hydrogen sorption at these pressures. With EFree support, Caltech researchers Nicholas Stadie, Brent Fultz, and their collaborators designed, constructed, and commissioned such an apparatus for thermodynamic gas adsorption measurements up to 70 MPa, a relevant target pressure for mobile storage applications. Materials such as zeolite-templated carbons (ZTCs) and carbon nanotubes that have drawn attention for showing unusual hydrogen sorption properties within this pressure regime are under investigation. An evaluation of ZTCs as potential hydrogen storage materials between 77 and 298 K up to 30 MPa was recently reported by the Caltech group. The uptake capacities of ZTCs remain the highest of known carbonaceous materials, but the nature of high-pressure adsorption in ZTCs is not unique despite their narrow microporosity and significantly lower skeletal densities. A linear relationship between surface area and hydrogen uptake at room temperature and 30 MPa was found across numerous carbon materials including ZTCs [N. Stadie et al., Langmuir 28 10057–10063 (2012)].
