Natural gas hydrates are an unusual form of ice-like material where a hydrogen-bonded network of water molecules traps methane molecules inside a cage-like framework. Deposits of these hydrates are stabilized by deep-ocean pressures in such vast amounts that they have long been considered to be a potential fuel source. However, a critical issue is understanding the stability limits of these structures, which decompose at ambient pressure. Fundamental to this aim is understanding how the gas molecules interact with each other and with the water molecules in forming their cages.
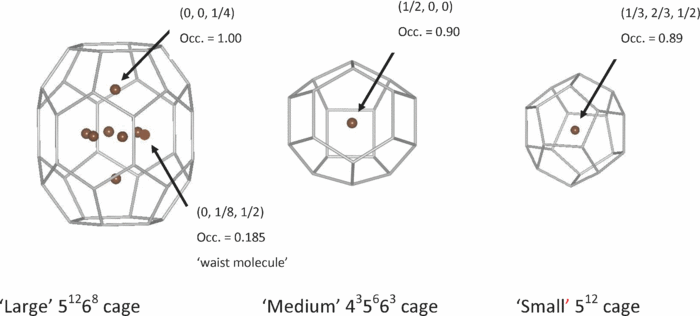
By using neutron diffraction measured at the high-pressure beamline of the Spallation Neutron Source (SNS) at Oak Ridge National Laboratory (ORNL), scientists including EFree scientist Malcolm Guthrie (Carnegie) and Chris Tulk (ORNL) and collaborators, have gained new insight on the structure of methane hydrate. State-of-the-art instrumentation at the SNS and a particularly pure sample permitted the first accurate determination of the number of methane molecules that can be trapped in their ice cages. This detailed information on the structure of the hydrate provides an ideal test case for theoretical models of the hydrophobic water-methane interaction potentials.
