 Carbon has long been a rich and active research area. It offers exciting discoveries of new allotropes including both crystalline and disordered structures such as buckyballs, carbon nanotubes, graphene, and diamond-like amorphous carbon with numerous and exciting potential in technological applications. However, the amorphous allotrope of diamond, i.e., the amorphous carbon based on complete sp3-bonding, has never been reported.
Carbon has long been a rich and active research area. It offers exciting discoveries of new allotropes including both crystalline and disordered structures such as buckyballs, carbon nanotubes, graphene, and diamond-like amorphous carbon with numerous and exciting potential in technological applications. However, the amorphous allotrope of diamond, i.e., the amorphous carbon based on complete sp3-bonding, has never been reported.A team of researchers from Stanford, Geophysical Laboratory, HPCAT, and HPSynC has discovered a new carbon allotrope at high pressure and room temperature with 100% sp3-bonding in bulk glassy form. Synchrotron inelastic x-ray scattering and x-ray diffraction probes revealed a pressure-induced sp2-to-sp3 bonding conversion in glassy carbon which was complete 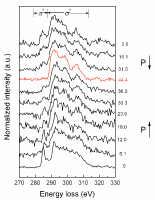

This amorphous, superhard carbon allotrope would have a potential advantage over diamond if its hardness turns out to be isotropic—that is, having hardness that is equally strong in all directions. The amorphous carbon created by Wendy Mao and Yu Lin (Stanford0 can be made thicker than the very thin films previously created by other researchers, and consequently, have more applications. In general, these research findings expand the wealth of pure carbon allotropes and open exciting possibilities for potential applications using superhard amorphous solids.
Top figure: Wendy Mao and Yu Lin (Stanford) loading samples into a diamond anvil cell.
Bottom figure: High pressure IXS carbon K-edge spectra of glassy carbon collected along the compression and decompression cycles. The red spectrum shows the complete σ-bonding in the new high pressure carbon phase. The numbers on the right side indicate pressure in GPa.
